1. General information
According to the Hungarian Reproductive Law fertility treatments can be provided to married couples and couples in a so-called common-law relationship. Both types of relationships need to be documented. In the case of common-law relationship the document has to state that the partners live in the same household, they are not married to anyone else and that the document is prepared for the purpose of fertility treatment. Furthermore, treatment can be provided to single women using anonymous donated sperm. Treatment can be offered based on proper medical indications and only after a detailed evaluation that is in accordance with the guidelines. Fertility treatments (intrauterine insemination, in vitro fertilization) that are performed based on an appropriate indication and after a proper evaluation are covered by the Hungarian Health insurance (NEAK). Fertility treatments can be only carried out in institutes that have a contract with the insurance company (NEAK). The upper age limit for insurance covered treatments is 45 years of age. Over the age of 45 only self-covered treatments can be performed.
2. The physiology of normal reproduction
2.1 The normal menstrual cycle
The first day of the menstrual bleeding is considered day 1 of a cycle. Cycles are considered regular when they occur every 21-35 days with not more than 1-2 days of difference. A typical cycle lasts for 2-7 days. A normal cycle can be divided into three phases: 1) menstruation, 2) follicle growth (follicular phase, proliferative phase) that ends with ovulation, 3) luteal phase (secretory phase) that ends with the next menstruation. In each cycle two events take place parallel with each other: 1) within the ovaries: follicle growth – ovulation – corpus luteum activity, 2) within the endometrium: endometrial build up (proliferation) – preparation for implantation (decidulaization) – menstruation (shedding the endometrium). At the end of each menstrual cycle as the activity of the corpus luteum declines and the hormonal support of the endometrium is lost, the menstruation will get started. In this phase, as the serum level of estradiol and progesterone (previously synthesized by the corpus luteum) decreases the pituitary secretion of follicle stimulating hormone (FSH) will increase. FSH will recruit a new cohort of follicles that start their journey to ovulation. It is always a group and not an individual follicle that starts to grow. The entire growth process (folliculogenesis) requires months to be completed; pituitary hormones FSH and luteinizing hormone (LH) only guide the last 2-2.5 weeks of the process. A cohort or wave of follicles start to develop together though the majority of them will never reach ovulation but arrest at various stages of the process. In a typical cycle, as a result of complex endocrine regulatory mechanisms, only 1-2 follicles reach the ovulatory stage. At this stage the size of the follicle is 22-25 mm in diameter. At the time of ovulation, the oocyte that is released from the follicle is picked up by the Fallopian tube where it could meet the sperm. Following ovulation, the hormone synthesizing capacity of the follicle (now called corpus luteum) changes too and its main secretory product will be progesterone. Leading up to ovulation the follicle produces increasing amounts of estradiol that is responsible for building up the endometrium. The progesterone, secreted by the corpus luteum, induces those qualitative changes that prepare the endometrium for implantation. If implantation does not occur the activity of the corpus luteum is maintained for 13-14 days. By the end of this period the level of estradiol and progesterone will be very low and will no longer be able to support the endometrium, therefore the menstruation is started. If implantation occurs, then the human chorionic gonadotropin (hCG) hormone produced by the embryo rescues the corpus luetum and it can maintain its activity.
2.2 Spontaneous fertilization
At the time of ovulation the egg (oocyte) is released from the follicle and the fimbriae at the end of the Fallopian tube will guide it into the tube. The peristaltic activity of the tube will move the egg further towards the uterus. If intercourse occurs around ovulation and sperm enter the uterus 24-36 hours after ovulation they may be able to fertilize the oocyte. During the process, first the sperm has to bind to the layer surrounding the egg and has to penetrate through it. Following successful fertilization, the fertilized egg starts to divide and develop and continues its way towards the uterine cavity. 5-7 days after fertilization the developing embryo now called blastocyst may implant if the endometrium is properly built up and is receptive.
2.3 Implantation
A successful implantation requires a healthy embryo and a properly built-up, receptive endometrium. The steps of implantation are guided by hundreds of up-, or down-regulated genes. It requires the presence of various adhesion molecules and the appropriate activity of the immune system is important too. The role of so-called uterine natural killer cells in the process of normal implantation needs to be mentioned. These cells are able to „communicate” with the embryo through chemical signals and therefore control the depth of implantation. The main reason for lack of or failed implantation is that the embryo that reaches the uterus is not healthy. As the age of the woman increases the proportion of embryos that are genetically not healthy and therefore cannot implant increases too. Successful implantation requires hormonal support that is provided by the corpus luteum and from gestational age week 6-8 onwards by the placenta.
3. Infertility
Successful reproduction requires regular ovulation, patent Fallopian tubes and sperm of sufficient number and quality. If these criteria are met and a young couple is sexually active around ovulation the chance of spontaneous conception is around 25% in the first month, is around 60-70% by the end of the first 6 months and is around 85% by the end of the first year. Infertility is diagnosed when pregnancy is not achieved by the couple after 1 year of attempts without contraception. In couples under 35 years an evaluation is warranted after 1 year; in couples over 35 the infertility work-up can be started after 6 months and in couples over 40 as soon as the couples asks for help. It is important to point out that the incidence of infertility is affected by age as well; under 30 about 8-10% of the couples are affected but by the age of 40, 30-40% of the couples may face difficulty conceiving.
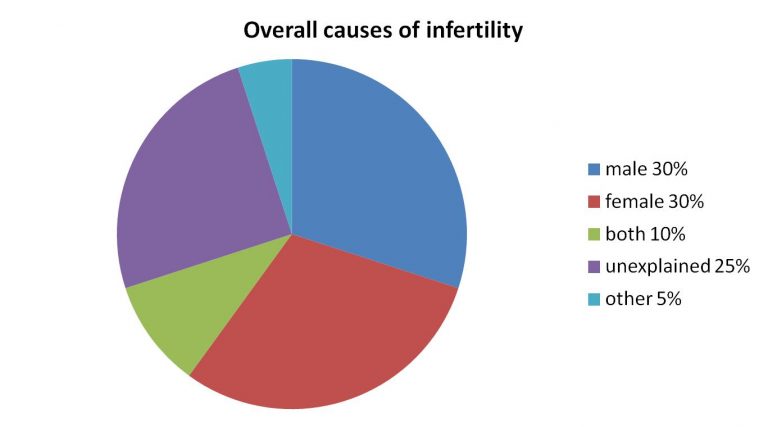
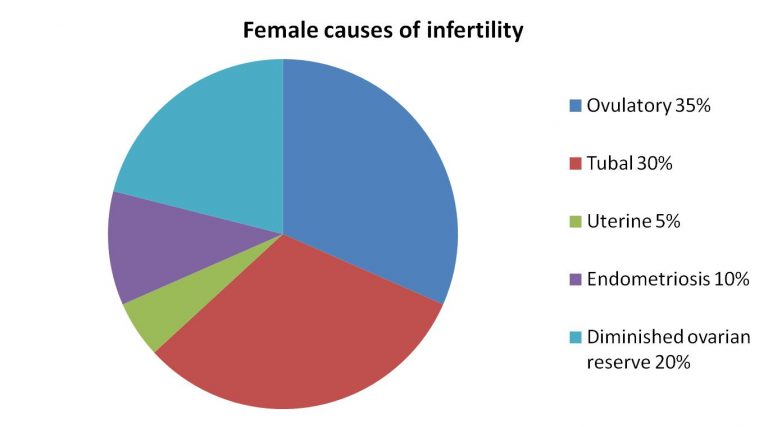
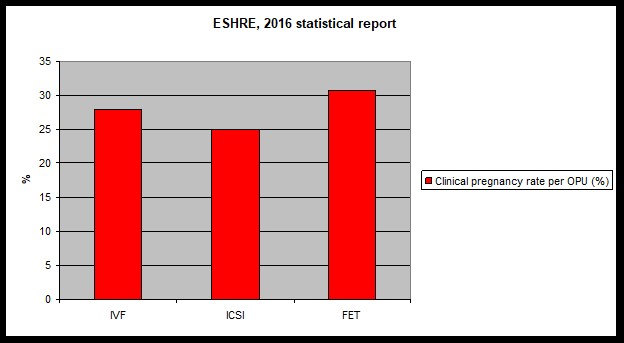
4. Spontaneous pregnancy loss, miscarriage
A miscarriage is defined as a pregnancy loss before the 24th week of gestation. A pregnancy can be diagnosed by detecting the hormone called hCG from urine or serum. When after an initial rise the hCG level plateaus and then starts to decline we talk about a biochemical pregnancy. A clinical pregnancy is diagnosed when a sac can be detected using ultrasound in the uterine cavity. In a normally progressing pregnancy, by the 5th week of gestation a yolk sac becomes visible in the gestational sac and subsequently the embryo and eventually the embryo with the cardiac activity will be seen. We talk about a missed abortion when embryonic development arrests but the embryo is not expelled from the uterus. The most common reason for a pregnancy loss is genetic abnormality affecting the embryo. The risk of pregnancy loss increases if the uterus has congenital defects, if endocrine abnormalities are present, in case of certain hematologic problems (primarily anti-phospholipid syndrome), in case of infections and as a result of certain medical problems, etc. About 3-5% of the pregnancy losses are recurrent pregnancy losses. In these cases, a minimum of two subsequent pregnancy losses are experienced. This condition warrants a proper evaluation.
5. Infertility evaluation
A basic work-up should include the following:
1) Hormonal assessment of ovarian function
2) Evaluation of the anatomic structures (uterus, uterine cavity, Fallopian tubes)
3) Assessment of sperm production
5.1. Assessment of ovarian function
When the ovarian function is evaluated, we want to confirm ovulation, look for endocrine abnormalities that could affect ovarian function (e.g.: thyroid disorder, overproduction of prolactin) and try to estimate the number of oocytes in the ovaries (ovarian reserve test). In order to assess ovarian reserve, we typically measure anti Müllerian hormone (AMH) level (values less than 1.1 ng/ml are considered low) or count the number of small follicles in the ovaries (antral follicle count). FSH and estradiol measurement at the onset of the cycle are still frequently used for the same purpose. The hormone test must be performed once between days 2-4 of the cycle. These test results can be used for counseling or for planning treatments. They do not provide information on oocyte quality, only about their quantity. Decisions not to start treatment are only made when the values indicate extremely low ovarian reserve.
5.2. Anatomic evaluation of the female genital tract
The evaluation of the anatomic structures involves a physical exam and some type of imaging study or rarely surgical findings can help with the correct diagnosis. Most commonly ultrasound, vaginal ultrasound, is used to assess the pelvic organs. Ultrasound can help to image the uterus, uterine wall, endometrium and ovaries and helps with the correct diagnosis of endometriosis related lesions. The uterine cavity can be assessed during saline sonohysterogram when physiologic saline solution is infused into the cavity to distend it, while the use of contrast liquid can outline the Fallopian tubes. An MRI may be needed to properly diagnose endometriosis, adenomyosis, fibroids or congenital anomalies. Laparoscopy and/ or hysteroscopy may be required to reach the correct diagnosis. Endometrial biopsy may be needed to obtain a tissue sample to look for inflammatory changes. Typically, a thin plastic catheter is used to sample the endometrium.
5.3. Andrologic evaluation
The first step of the examination of the male partner is a semen analysis. Quantitative and qualitative analysis of the semen specimen, provided after 3-5 days of sexual abstinence, is performed based on the WHO Guidelines. Repeated sperm analysis 4-6 weeks later, hormonal analysis and genetic testing may be offered if the sperm parameters are below the standards. The evaluation of the male partner is completed by a general andrological exam. This includes obtaining a detailed case history (retentio testis, cryptorchidsm, malformations, urological diseases etc.), a physical examination (general physical status as well as examination of the genitals [volume of testicles, status of epidydimis etc.]) and ultrasound scanning if indicated.
Certain normal sperm functional parameters are required for fertility in addition to normal traditional sperm parameters (concentration, motility, normal morphology). Mature spermatozoa are formed from germinal cells via mitotic and meiotic divisions and then undergo post maturation in the epidydimis. As a result of further changes (capacitation, acrosome reaction) that happen in female genital tract, spermatozoa become capable to fertilize the egg. During fertilization, spermatozoa transmit not only genetic material to the oocyte but also an activation factor, centrosome and a host of messenger RNA. These steps are carried out in vitro when assisted reproduction is performed. Sperm preparation is traditionally done by the so-called density gradient centrifugation and/or swim-up technologies.
5.4. Sperm functional tests
Nowadays, the semen analysis is often completed with different functional tests:
- Genetic analysis of spermatozoa (SAT, Sperm Aneuploidy Test): Proportion of aneuploid spermatozoa (genetically unhealthy sperm) is measured when numerical chromosome aberration is detected (one or more chromosomes are missing or extra chromosome(s) is/are present). It is an expensive test and not significant for most patients.
- Detection of DNA fragmentation index (SDFI): Fragmentation of sperm DNA is thought to be more significant. During the test the proportion of spermatozoa with fragmented DNA is detected. The higher the SDFI is the lower the chance of a successful pregnancy is. Based on recent studies, efficacy of intrauterine insemination (IUI) is very low when the SDFI exceeds 25 %. It is recommended to apply ICSI when SDFI is higher than 30-35 %. DNA fragmentation is correlated with different clinical problems or life-style factors (infection, fever, elevated temperature around the genital tract, certain occupations, tight pants, elevated age, varicocele, smoking, drugs, and pollution). Identifying and treating these issues can reduce DNA fragmentation. There are however no effective selection technologies available that have proven efficacy to filter out spermatozoa with damaged DNA. PICSI method (physiological sperm injection) is often used for this purpose; however clinical studies have not confirmed its efficacy yet. Recent publications regarding a microfluidic device produced by ZYMOT (Fertile Plus) suggested its efficacy, but randomized clinical trials (RCT) are still missing.
- Acrosome reaction test (AR): Acrosome reaction occurs under physiological conditions when spermatozoa are attached to the outer layer of the ovum. This step is required for sperm to be able to penetrate into the egg. Spontaneous acrosome reaction (AR) happens in a proportion of spermatozoa both in vivo and in vitro. High incidence of spontaneous acrosome reaction may however cause a problem at the time of fertilization. Acrosome reaction can also be induced by chemical agents (ARIC score = AR % in the induced sample). Low ARIC score is associated with low chance of fertilization. AR test is not evidence based, thus it is not widely used in routine andrology.
- Measurement of reactive oxygen species (ROS): Reactive oxygen species play role in DNA fragmentation. A small amount of ROS is needed for sperm functions, however high concentration correlates with male infertility.
- Measurement of static oxidation-reduction potential (sORP), MYOXSYS System: Static oxidation-reduction potential of fresh or frozen-thawed sperm sample can be detected with the MYOXSYS system. It can help the work-up of the following clinical problems: repeated miscarriage (RPL), repeated implantation failure (RIF), elevated paternal age, unexplained infertility, infections of the genitals, varicocele, life style risk factors, and low sperm count of unknown etiology.
- Quantitative measurement of cytokines: Quantitative measurement of cytokines (IL-6, IL-8) is useful in diagnosis of hidden inflammation of the genitals.
- Hyaluronan binding assay (HBA): (HBA): Hyaluronan binding assay is widely used in practice. Hyaluronan is a natural component of the cell layer (granulosa cell matrix) that surrounds the ovum. Initial studies (Huszár et al.) have suggested that most of the mature and genetically normal spermatozoa can bind to hyaluronan, thus it was thought that HBA test could be used to select the adequate treatment (IUI, IVF, ICSI). Meta-analysis of the studies (RCT) however has failed to confirm the efficacy of HBA test. It is not evidence based either that the HBA test is able to assess the fertilizing potential of spermatozoa. Its use may be considered in cases of repeated fertilization failure.
6. The evaluation of „recurrent implantation failure
The success of IVF primarily depends on the age of the woman. The success rate rarely exceeds 50% even in the youngest age group. If a treatment fails, typically a new cycle (either a complete new cycle or a frozen embryo transfer cycle) is initiated. Failed implantation may be related to the embryo, to the endometrium or to poor transfer technology. A certain proportion of the embryos conceived in vivo or in vitro are chromosomally abnormal. At the age of 30, 20-30% of the embryos are affected; by 35 about half of them and by age 40 as many as 70% of them could be abnormal. By the age of 45 it is rare for a chromosomally normal embryo to be conceived. In addition to maternal age, the quality of the sperm has a great impact on embryo quality. From age 40 onwards the proportion of abnormal sperm (aneuploidy, increased DNA fragmentation) increases. Therefore, in the majority of the cases failed implantation is the result of poor embryo quality. Most chromosome errors occur spontaneously and are not repetitive. They may affect a single chromosome or multiple within the same embryo. One study reported that the cumulative pregnancy rate after the transfer of three genetically tested embryos (single embryo transfers repeated three times if needed) could reach as high as 95%. There is no generally accepted definition of recurrent implantation failure (RIF) but many consider the lack of pregnancy after the transfer of 4-5 good morphology cleavage stage embryos or 3 good morphology blastocysts as RIF. It is also important to mention that the problem is not necessarily recurrent as each failure may have a different etiology. Numerous tests as work-up and multiple interventions as treatment have been evaluated in cases of RIF. Genetic testing of the couple may reveal chromosome errors (e.g.: balanced translocation) that may raise the proportion of chromosomally abnormal embryos. In such cases, preimplantation genetic testing of the embryos could identify those not affected that could be transferred. Testing of patients with RIF may reveal a higher incidence of hematologic problems but the use of blood thinners has not been shown to improve reproductive success. The currently available serum tests for immunologic dysfunction are unable to establish a diagnosis or serve as the basis for treatment. It is important to evaluate the uterine cavity while laparoscopy may identify disorders (endometriosis, inflamed Fallopian tubes) that could be operated on to improve success rates. Histologic evaluation of the endometrium could identify inflammation of the endometrium (endometritis) and its treatment could also increase the chance of implantation. Endometrial scratching itself does not seem to improve implantation rates according to recent clinical trials though the majority of the patients enrolled underwent their first or second IVF treatment and were not necessarily patients with RIF. Sperm selection methods (e.g.: PICSI) prior to ICSI have not been associated with better treatment outcome. Culturing the embryos to day 5, the blastocyst stage could be helpful though. Genetic testing of the embryos for aneuploidy may serve with benefits, though this technology is not allowed in Hungary at this stage. According to published evidence the use of endometrial receptivity assay (ERA) does not increase the chance of implantation. In true cases of RIF, an evidence-based evaluation is warranted and then a new transfer may follow hoping that an embryo conceived with a different oocyte and sperm that is transferred into a newly built, different endometrium will implant.
7. The evaluation of recurrent miscarriages
A certain proportion of embryos conceived spontaneously or through assisted reproduction technology arrest after implantation and are miscarried. The majority of the losses are preclinical; they are lost before a test could detect them. About 20-25% of the clinically diagnosed pregnancies are still aborted. The proportion is lower (about 10%) among younger patients but could affect as many as 50% of the pregnancies by the age of 45. A chromosome abnormality is the main cause of these sporadic losses; in such cases an unhealthy embryo implants that is eventually identified and is rejected by the body. About 3-5% of the losses are recurrent and in these cases 2-3 or more losses follow each other. An evaluation is warranted in such cases. Unfortunately, these „investigations” in routine practice often include tests that are not related to pregnancy losses and therefore cannot be used to formulate a therapeutic plan based on them. The risk of pregnancy loss increases if one of the parents carries a chromosome abnormality, if anti-phospholipid antibodies are detected (hematologic disorder), if the uterine cavity is deformed, if the proportion of sperm with DNA fragmentation is elevated, if the body mass index is high, in the presence of chronic endometritis, among smokers and in case of certain endocrine disorders (e.g.: thyroid dysfunction). These problems should be looked for and treated if diagnosed. It is important to mention that in about half of the recurrent pregnancy loss cases no cause can be identified. It is also important to explain to patients with unexplained recurrent pregnancy loss that the long-term prognosis is not necessarily poor as the majority of the couples regardless of the number of previous losses will eventually be successful.
8. Uterine anomalies
The uterus is built up of muscles and connective tissue. The size and position of the uterus varies. The uterine cavity is covered by endometrium that undergoes cyclic changes unless an embryo successfully implants. The various disorders of the uterus may affect the wall of the uterus or the endometrium and could be congenital or acquired postnatal. During embryonic life the uterus develops as two halves that fuse later on, and the dividing membrane is subsequently absorbed. Problems with these processes may result in congenital anomalies. These anomalies range from complete duplication of the uterus to just a minimal indentation at the top of the cavity. Congenital anomalies may result in difficulty conceiving, could increase the risk of pregnancy loss or preterm delivery. Some of these anomalies (e.g.: a uterine septum that divides the cavity) can surgically be corrected. Since not all anomalies require surgical correction, the precise diagnosis using imaging technology or even diagnostic surgery is important. One should keep in mind to evaluate the urinary tract as well in these cases as urinary tract abnormalities are common when uterine abnormalities are diagnosed. Finally, in cases of uterine abnormalities it is even more important to aim for a singleton pregnancy to reduce the risk of preterm deliveries.
Uterine fibroids and adenomyosis are the most important acquired uterine anomalies. Fibroids are benign tumors that originate from the muscular layer of the uterine wall. They could be solitary or multiple and may grow towards the uterine cavity, the abdominal cavity or may be confined mostly to the uterine wall. Their impact on reproduction increases with size and number. They may block the Fallopian tubes, may deform the cavity, may interfere with proper endometrial build-up, may interrupt normal blood flow to the endometrium and could interfere with the contractility of the uterus. Fibroids that distort the cavity should be surgically removed. Removal of those fibroids that grow towards the peritoneal cavity is not necessary. In cases of fibroids that are primarily located in the uterine wall, the decision is made individually and is based on their size and number. It is hard to establish universal management guidelines; while it has been shown that fibroids lower the chance of implantation, studies have not shown higher pregnancy rates after surgical removal.
Adenomyosis is diagnosed when endometriosis infiltrates the uterine wall. Definitive diagnosis can be made based on MRI findings, but various ultrasound signs could indicate the presence of adenomyosis. Adenomyosis usually diffusely infiltrates the uterine wall and therefore surgical removal is not possible. Rarely it forms a well-defined ball-like lesion that could be removed surgically. Hormonal treatment may reduce adenomyosis and therefore could improve implantation rates but well-designed studies should evaluate the exact role of medical treatments to help clinical decisions. Hormonal treatment can be considered when the adenomyosis results in the deformity of the uterine cavity and previous fertility treatments have failed or in cases of recurrent pregnancy losses that are not explained otherwise.
Endometrial polyps form when focally the endometrium proliferates without proper control. They are mostly smooth lesions of different sizes. Polyps may interfere with implantation or could be responsible for pregnancy loss and therefore their surgical removal is indicated especially if they are over 1 cm in size.
9. Endometriosis
Endometriosis is diagnosed when tissue that is similar to the endometrium can be found outside of the uterine cavity. Most commonly it can be diagnosed along the Fallopian tubes, on the ovaries or behind the uterus but rarely it can be found in distant locations too. It affects 10-15% of women but could be detected in up to 1/3 of infertile women. There is a wide variety of symptoms that can accompany endometriosis and there are cases when it is completely asymptomatic and is found only during surgery. Endometriosis is associated with lower pregnancy rates. This is partly caused by a negative effect on oocyte quality, partly via mechanisms that interfere with normal implantation. The stage of the disease depends on how extensively the endometriosis spreads, how many organs are involved and the density of the accompanying adhesions. Surgical treatment of early stage (I-II) disease could improve spontaneous pregnancy rates. In more advanced cases (stage III-IV) typically in vitro fertilization is recommended. Surgical treatment of endometriosis prior to IVF is recommended to those who have significant symptoms associated with the disease that negatively impact on everyday quality of life. Surgery is also recommended in those cases when the bowel lumen or the ureters are obstructed. In cases of larger or multiple ovarian cysts surgery can be considered but the ultimate decision needs to be made taking the ovarian reserve into account too. Medical treatment of symptoms alone in otherwise infertile women is not recommended as it further delays a pregnancy. In some cases, short medical treatment is offered preceding an IVF treatment.
10. Infertility treatments
10.1. Life-style changes
Most of the parameters that influence infertility treatment outcome (e.g.: age, ovarian reserve, sperm parameters) cannot be influenced. There are a few exceptions and in these cases the patient may positively influence the outcome of her treatment. Smoking is known to be associated with multiple adverse effects. It is however less known that the toxins in cigarette smoke have a negative effect on eggs and sperm. Smoking in men increases the instability of sperm DNA (DNA fragmentation) and is associated with slower sperm motility. As a result of these adverse effects, the risk of infertility is higher and the risk of pregnancy loss is more common too. Smoking has harmful effects on the oocytes too. The loss of oocytes is accelerated and menopause is reached 1-4 years earlier. This disadvantage is seen at a younger age too and the diagnosis of low ovarian reserve is more common. The risk of miscarriage and extrauterine pregnancy is higher too. A higher body mass index (BMI) has also been linked to reduced fertility treatment efficacy. Typically, higher medication dose is required during stimulation, fewer oocytes are collected during IVF and implantation rates are lower too. In case of high BMI as little as 5% weight loss could already have a positive impact. A calorie restricted, well-balanced diet, regular physical activity and if needed weight loss medications could help this process. Low to moderate alcohol consumption does not seem to have an effect on stimulation outcome or implantation but even small amounts of alcohol can have harmful fetal effects during pregnancy. Alcohol consumption therefore is advised against after embryo transfer. Regular physical activity can help maintain a normal body habitus, could be helpful when weight loss is desired, improves circulation and therefore could positively influence implantation. Too intensive physical activity can however disrupt the menstrual cycles. Mild to moderate physical activity maintained during pregnancy does not seem to be associated with poorer pregnancy outcome. In general, a Mediterranean type diet is recommended to infertile couples. Women of reproductive age should daily consume 400 mcg folic acid and during fall-winter when sunshine is less vitamin D supplementation is recommended.
10.2. Ovulation induction
Some women are affected by irregular ovarian activity or absence of ovarian cycles. In their case, induction of follicular development (ovulation induction) alone often restores the ability to conceive. This usually means pharmacologic interventions but in some cases life-style changes (diet, weight loss) only may restore cyclic ovarian activity. The medications used in such cases either stimulate the ovaries (anti-estrogens: clomiphene citrate, tamoxifen; aromatase inhibitors: anastrazole, letrozole; gonadotropin injections) directly or correct the underlying endocrine abnormality to restore cyclic ovarian function (treatment of thyroid dysfunction or elevated prolactin levels). The goal of treatment in these cases is to restore monofollicular development.
10.3. Stimulation and timed intercourse
This treatment is offered to those who otherwise ovulate regularly but through the induction of multiple follicles and through the availability of multiple oocytes for spontaneous fertilization we wish to increase the chance of pregnancy. There are several stimulations options in these cases too: anti-estrogens: clomiphene citrate, tamoxifen; aromatase inhibitors: anastrazole, letrozole; gonadotropin injections. In a stimulated cycle we do not aim for the simultaneous growth of more than 3 follicles to minimize the chance of multiple pregnancy. Once the follicles reach maturity, we either wait for spontaneous ovulation or use a trigger injection to induce it. The chance of conception is highest during the 12-36 hours after the trigger injection.
10.4. Natural cycle or stimulated cycle + insemination
This treatment is offered to those who otherwise ovulate regularly but the chance of pregnancy is increased through the induction of multiple follicles and through the availability of multiple oocytes. There are several stimulations options in these cases too: anti-estrogens: clomiphene citrate, tamoxifen; aromatase inhibitors: anastrazole, letrozole; gonadotropin injections. In a stimulated cycle we do not aim for the simultaneous growth of more than 3 follicles to minimize the chance of multiple pregnancy. Once the follicles reach maturity, we either wait for spontaneous ovulation or use a trigger injection to induce it. The insemination is performed 36-40 hours after the trigger injection. On the day of the insemination the partner produces a semen sample that is processed and prepared for the insemination. During the insemination itself, a thin, soft plastic catheter is used to inject the processed semen into the uterine cavity.
In vitro fertilization (discussed separately).
11. In vitro fertilization
11.1. Stimulation for IVF
The ideal oocyte yield during IVF is between 10-15 oocytes. When more oocytes are retrieved the risk of so-called ovarian hyperstimulation syndrome (OHSS) increases, while with fewer eggs our ability to select the embryo(s) for the transfer and the chance for cryopreservation decrease. The stimulation protocol and the type and dose of the medications used during IVF are individually determined. Individualization depends on age, ovarian reserve markers, ultrasound findings and response to previous treatment if available. The stimulation is started on day 2 or 3 of the spontaneous cycle or following medical preparation using contraceptive pills, GnRH agonist injections or estradiol. Almost all injections are subcutaneous that can be self-administered after a quick tutorial. During stimulation medications that promote follicle growth as well as medications that prevent premature ovulation are used. The response to treatment needs to be monitored using ultrasound and if needed blood tests. Once the leading follicle(s) have reached an adequate size the process of ovulation is induced with a trigger injection and 35-36 hours later the oocyte retrieval is performed. Alternatively, stimulation can get started at any phase of the cycle or even twice within one cycle. In such cases however the embryos need to be cryopreserved and can only be transferred later on.
11.2. Egg retrieval
Egg retrieval (follicular aspiration, puncture) is a short surgical procedure (10-15 minutes) usually done under anesthesia. Mature (metaphase II) and immature oocytes can both be found among the retrieved eggs. The structure of the granulosa cells around the egg (cumulus oophorus complex, COC) provides information about the maturity. 20-30% of the eggs are immature; their COC is less expanded. The relatively high incidence of immature eggs can be explained by multiple follicular development after stimulation. Some of the immature eggs can spontaneously complete maturation or the maturation process can be induced in vitro (in vitro maturation [IVM]). Maturity of eggs strongly correlates with the efficacy of the IVF treatment. Embryonic development is compromised and pregnancy rates are lower when the eggs are immature. The embryos at various stages of their development have different nutritional needs. These nutritional needs are provided through the different composition of the applied culture media. Eggs and embryos are cultured in incubators. The incubators provide the optimal temperature (37 °C) and atmosphere for proper embryonic development. Fertilization is done a few hours (2-5) after the egg retrieval. The morning after the fertilization (16-18 hours after fertilization) we confirm successful fertilization using a microscope exam. Normally fertilized eggs (2PN) are separated from unfertilized and abnormally fertilized (triploid) ones. Process of fertilization can be delayed, especially in case of immature eggs, thus the first assessment does not necessarily show the final status. In most IVF labs embryos are cultured in microdroplets (20-50 µL droplets of culture media covered by paraffin oil). Typically several embryos are cultured together as group culture has beneficial effects on embryonic development. The development of embryos is checked every 24-48 hours. The selection of embryo(s) for transfer is done on Day3 or Day5 after egg retrieval in the majority of cases.
11.3. In vitro fertilization
During traditional IVF prepared and diluted suspension of sperm (100.000-150.000 spermatozoa/egg) is added to the culture medium. The subsequent events of fertilization are identical to the physiological process. Sperm cells pass through the matrix of granulosa cells and then attach to the outer layer (zona pellucida) of the egg. Penetration through the zona pellucida requires enzymes and intense movement of the sperm. Once the sperm has entered the cytoplasm, it induces biochemical events that block penetration of another sperm.
11.4. Intracytoplasmic sperm injection (ICSI)
In case of suboptimal sperm parameters (low concentration, inadequate motility etc.) or in cases when spontaneous fertilization is unlikely to happen, sperms can be directly injected into the egg’s cytoplasm with the aid of micromanipulator. This procedure is called intracytoplasmic sperm injection. Morphologically normal and motile sperm is selected for injection by the biologist. Sometimes there aren’t any motile or morphologically normal sperm in the sample. This doesn’t rule out the possibility of successful fertilization. Morphologically abnormal spermatozoa are capable of fertilizing the egg if it has intact genome, centrosome and a functional activating factor. Immotile but viable sperms can be selected with adequate selection method (hypoosmotic swelling test, HOST).
11.5. ICSI with surgically retrieved sperms (TESE, MESA)
Azoospermia is diagnosed when sperm cannot be found in the semen at all. If repeated semen analysis confirms azoospermia, sperm may still be surgically retrieved from the testicles (TESE) or the epididymis (MESA). They can be used to fertilize the oocytes using ICSI. In some cases of azoospermia (complete maturation arrest), morphologically mature sperm can’t be found in the biopsy sample. Certain types of premature sperm cells (elongated spermatid, round spermatid) can be used for fertilization as these forms have haploid genome. Efficacy of the injection of elongated spermatids (ELSI) and round spermatids (ROSI) is well below to that achieved with mature sperms.
11.6. Sperm selection with microfluidic device (Fertile Plus)
The use of a special microfluidic chip (Fertile Plus by ZYMOT) allows a more gentle preparation of semen compared to the traditional methods (density gradient centrifugation). The chip contains a membrane that separates the semen from the culture medium. Motile sperms swim up through the pores of this membrane. It provides a more gentle separation that eliminates the extensive oxidative stress of centrifugation. Currently there isn’t enough scientific evidence supporting a widespread use of the Fertile Plus Chip in ART (IUI, IVF) since it is a relatively new product. Some articles based on studies performed in limited number of patients have suggested that the chip may be effective in selecting sperm with intact DNA.
11.7. Sperm selection based on hyaluronan binding of sperms (PICSI)
Physiologic intracytoplasmic sperm injection (PICSI) is based on hyaluronic acid (HA) binding capacity of spermatozoa. HA is an element of the matrix connecting granulosa cells around the egg and it plays a role in natural selection of spermatozoa. Only mature sperm are able to attach to the HA layer covering the bottom of the PICSI dish. At the time of its introduction in 2005 it was suggested that the injection of HA-bound spermatozoa increases the efficacy of ICSI. However, the efficacy of PICSI to improve fertilization and pregnancy rate still hasn’t been confirmed in clinical trials. There are two studies in which the authors have found reduced miscarriage rate in cycle where PICSI selected sperm was used for fertilization. It hasn’t been confirmed either that PICSI could select sperm based on DNA fragmentation.
11.8. Embryo transfer (ET)
Embryos are transferred to the uterus using a thin plastic catheter. The transfer is usually a short, painless procedure. In most IVF cycles multiple embryos are created and the best 1-2 embryos are selected using specific criteria (speed of development, morphology). It is extremely important to offer the best transfer circumstances (day of transfer [2, 3, 4, or 5] and number of embryo(s) transferred) to the patient. Day 2 and Day 3 transfers had been performed in the past before adequate culture media for extended culture of embryos was developed by the end of 1990s. Transfer of blastocyst stage embryos on the fifth day improves the chance of implantation. Blastocyst stage ET is generally recommended if there are at least three good quality, 6-8 cell embryos on day 3 of culture. Progress from day 3 to day 5, to the blastocyst stage cannot be guaranteed though. In other cases (e.g.: multiple failed IVF previously), a blastocyst stage transfer can be tried intentionally even if there are fewer good quality embryos early on. On the other hand, an earlier embryo transfer (Day2, Day3) is recommended when a patient’s embryos can’t adapt to the artificial conditions and an adverse effect of prolonged culture is seen during previous attempts. The number of embryos transferred should be determined based on the patient’s age, previous history, parameters of the cycle, developmental stage and quality of the embryos. The goal is to achieve a singleton pregnancy.
11.9. Assisted hatching
Hardening of zona pellucida can happen during embryo culture. It can interfere with implantation after the embryo transfer. To aid implantation in these cases, a gap is created in the zona. Nowadays it is done using microsurgical laser. Assisted hatching is recommended if: thick zona (>15 µm), elevated maternal age (>35 years), elevated basal FSH-level, repeated implantation failure. Professional application of laser doesn’t affect the viability of embryos. However, some articles have suggested a correlation between monozygotic twinning and assisted hatching.
11.10. Cryopreservation of embryos, frozen embryo transfer (FET)
In most IVF cycles more embryos are created than transferred. Cryopreservation allows us to store and transfer these embryos later on. Thus, there is no need for another full IVF cycle. Early on the so-called slow freezing (computer controlled freezing) technology was used. Later a new method, named vitrification took over as the method of cryopreservation. The essence of vitrification is to immerse a small sample (1-2 µL) directly to liquid nitrogen (-196 °C) or supercooled air. Cooling of the minute sample is so fast that water molecules can’t form ice crystals but harden into glass-like structure. This is why this method is called vitrification. Survival rate of vitrified-warmed embryos is more than 90 % compared to 50-60 % efficacy of traditional freezing. The pregnancy rate achieved with the transfer of vitrified embryos is the same or even better than that achieved following fresh embryo transfer.
12. Gamete donation, embryo donation
Gamete or embryo donation are fertility treatment options when own gametes (egg, sperm) aren’t available for ART (IUI, IVF).
12.1. Sperm donation
Sperm donation has been widely used for decades. Sperm donation is legally regulated in Hungary. There are domestic sperm banks that use up-to-date technology for cryopreservation of semen from screened donors. Screening covers tests for infectious diseases, family history, andrological, genetic and psychological examination. Tests for infectious diseases are repeated half year after the last sperm donation. Previously frozen samples are only released to be used if the repeat tests are negative too. The selection of donor samples is usually based on phenotypic similarity of the donor. In Hungary, only samples from anonymous donors are allowed to be used. The selected sample is shipped to the IVF center in frozen condition. Thawing of the sample is done at the time of the ART treatment (IUI, IVF).
12.2. Egg donation
Egg donation requires more serious coordination especially when freshly retrieved eggs are used. The cycles of the donor and the recipient need to be synchronized. The use of cryopreserved eggs is an alternative option. Since the introduction of vitrification, the efficacy of IVF with cryopreserved eggs is comparable to the success of fresh cycles. Egg donation is allowed anonymously or between relatives when the offer of oocytes is directed. Unfortunately, anonymous donation doesn’t work perfectly due to the complicated regulations.
12.3. Embryo donation
Embryo donation is possible if a couple undergoing fertility treatment offers their excess embryos for donation. In this case it’s neither required and nor possible to perform all the tests that are normally performed in case of gamete donation. The mandatory tests prior to embryo donation are listed in professional guidelines.
13. Preimplantation genetic diagnosis and screening (PGT-SR/PGD, PGT-A/PGS)
In vitro fertilization allows genetic testing of the embryos prior transfer (PGD). Genetic analysis of the first and second polar bodies (PBB) provides information about the oocyte itself, while examination of blastomeres (BB) or trophectoderm cells (TEB) provides information about the embryo. In order to perform the analysis, a sample to be tested needs to be obtained. For this, first the zona pellucida is opened with microsurgical laser and then the polar bodies, blastomeres or trophectoderm cells are removed with special micropipettes. The biopsy for the first polar body is performed before fertilization while the second polar body can be removed the next day, after fertilization. Embryo biopsy is performed on Day3 or Day5/Day6 depending on the type of molecular genetic test used to assess the chromosome content of the embryos. One or two blastomere(s) are removed at Day3 biopsy. Trophectoderm cells, that later form the placenta, are biopsied on Day5/Day6 in case of blastocyst biopsy.
Preimlantation genetic diagnosis (PGT-SR, PGT-M or PGD) is performed if the parents carry a known genetic disorder (numerical or structural chromosome aberration or monogenic disorder). In case of preimplantation genetic screening (PGT-SR or PGS) the parents do not have a known genetic disorder; in these cases we wish to identify the healthy, euploid embryos. Theoretically, higher pregnancy rate should be achieved with the transfer of euploid embryos but the scientific evidence is rather conflicting regarding this issue. Thus PGS is not allowed for clinical use in Hungary.
Traditionally fluorescence in situ hybridization (FISH) has been used to detect numerical and structural chromosome disorders and polymerase chain reaction (PCR) to test for monogenic disorders. The molecular, biochemical diagnostic tools have rapidly improved since. Comparative genomic hybridization (CGH, aCGH) and next generation sequencing (NGS) are recommended for PGS in case of repeated miscarriage (RLP) or implantation failure (RIF) and elevated maternal age.
Samples from cleavage stage embryos and even from blastocysts contain only a limited number of cells, thus the diagnostic accuracy is not as reliable as with routine cytogenetic analysis. Therefore, prenatal genetic examination is recommended after PGD or PGS.
14. Complications of assisted reproductive technology
IVF in general is a low-risk intervention. This is partly due to the nature of procedure and partly to the young and mostly healthy patient population we treat. We however cannot forget that risk-free interventions do not exist. During the stimulation phase of the treatment multiple follicles develop and produce much higher estradiol levels when compared to a natural cycle. This carries an increased thrombosis risk. Overall, however, the risk is so low that preventive anti-coagulation is not required. Patients who are at a higher baseline risk for thrombosis should receive preventive anticoagulation though.
Injections site reaction or allergy to the preparations used during IVF are rare.
Some patients are sensitive to stimulation and produce more than expected number of follicles. If on top of their excessive response they become pregnant as a result of the treatment they are at risk for developing severe ovarian hyperstimulation syndrome (OHSS). As part of OHSS the ovaries become enlarged, the abdomen gets distended and significant pain, nausea and vomiting may accompany it. The ovaries often secrete fluid into the abdominal cavity and as the fluid accumulates it may add to the abdominal discomfort. In even more severe cases, the patient could develop shortness of breath, circulatory and renal function problems may develop and the risk of thromboembolism could become significant. Once OHSS develops treatment to ease the symptoms can be offered. Prevention of OHSS is therefore important; those at risk need to be identified, they need to be treated using proper stimulation protocols, medication regimens and if despite these measures there are still signs of developing OHSS they need to have their embryos cryopreserved to avoid a pregnancy. Embryos can be transferred later on once the symptoms have disappeared.
The risk of intraoperative complications (bleeding, bladder/ bowel injury after the retrieval) is well under 1% of the cases. If they occur though, they may require further surgical treatment.
Nowadays most experts consider the singleton pregnancy as the good outcome after ART and therefore a multiple pregnancy (especially higher order multiple pregnancy) is considered a „complication”. In 1-2% of the pregnancies the embryo implants outside of the uterus and an ectopic pregnancy is achieved and very rarely simultaneous intra-, and extrauterine implantation is seen. These cases require surgical treatment.
15. Congenital anomalies after assisted reproduction
Congenital anomalies can be found in 2-3% of the pregnancies in the general population. Infertile women differ regarding multiple parameters from those in the general population and among them the risk of congenital anomalies is somewhat higher. Infertility itself is associated with an increased risk since a higher incidence of anomalies is seen after intrauterine inseminations and ovulation induction too. During IVF the gametes, embryos spend part of their development in the lab outside of the maternal body and often are exposed to laboratory interventions. Therefore, it is reasonable to evaluate whether these interventions carry any extra risks. Following IVF, the risk of congenital anomalies is relatively (by 20-30%) higher compared to that observed in spontaneously conceived pregnancies. This means that the risk is 3-4% instead of the expected 2-3%. The risk is the highest in couples who undergo ICSI treatment to treat severe male factor infertility. The risk of congenital anomalies is also higher in multiple pregnancies that are more common after ART. It is recommended that all patients who conceive through ART treatment to undergo the recommended prenatal genetic screening.
According to current knowledge there are no significant long-term risks. For certain gynecologic cancers infertility is a risk factor but the use of stimulation does not further increase this risk.
16. Success rates
A fertility treatment is considered successful when it ends with the birth of a healthy, full-term singleton. Any other „success” is just a surrogate marker of success (number of oocytes, embryos, embryo quality, positive pregnancy test, etc.).
Success is determined by numerous factors among which maternal age is the most important. As the age increases the ovarian oocyte supply decreases and the oocyte quality declines too. Poor quality oocytes are more likely to result in a chromosomally abnormal embryo that will not implant. The proportion of abnormal embryos is 20-25% at age 30, it reaches 60-70% by age 40 and exceeds 90% by the time a woman reaches 45.
We cannot ignore the impact of male age either as from age 40 onwards the quantitative and qualitative sperm parameters decline too.
Besides female age we also need to consider the ovarian reserve. Few oocytes collected after a difficult stimulation are less likely to result in an embryo that can implant.
We need to consider the number and quality of the embryos. The uterus has to be healthy and a proper endometrium is required for successful implantation.
As the BMI increases the chance of implantation decreases. The different gynecologic problems (fibroids, endometriosis) may interfere with reproductive success.
Finally, it is important to point out that the chance of a healthy young (<30 years) couple to be successful during the first month when they try to conceive is 25-30%. The cumulative chance increases to 60-70% by six months and to 85-90% by the end of the first year. IVF success rates should be evaluated in light of these figures and by understanding that there are no two identical cases limiting the comparability of results.
When success rates are evaluated it is important to see whether they are reported per started cycles, per retrieval or per embryo transfer. As the success of cryopreservation technology has improved an increasing proportion of cycles ended in elective cryopreservation for various reasons. In these cases, the lack of transfer is not due to not having an embryo but is due to the decision to cryopreserve them. Therefore, if the pregnancy rate is reported per stimulation or per retrieval, we may not get the right impression about the level of work in that clinic. Hence, two ways or reporting are recommended in general: 1) pregnancy/ live birth rate per embryo transfer or 2) cumulative pregnancy/ live birth rate per started cycle, after the transfer of all available embryos fresh or frozen. Furthermore, it is advised to compare patients with similar baseline characteristics. This, as a minimum means the comparison of success rates by age groups or by treatment number.
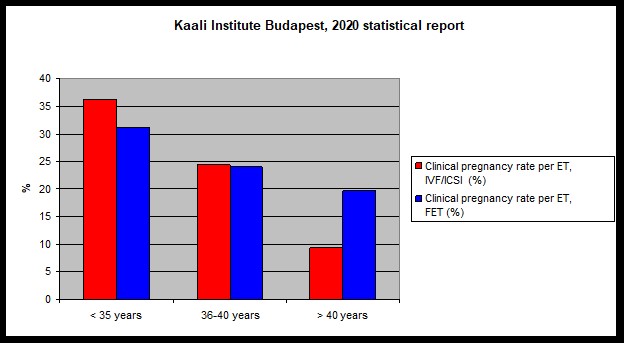
In 2020, 10.7% of pregnancies following IVF-ICSI were twins and there were no triplet pregnancies.
European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE), Wyns C, Bergh C, Calhaz-Jorge C, De Geyter C, Kupka MS, Motrenko T, Rugescu I, Smeenk J, Tandler-Schneider A, Vidakovic S, Goossens V. Hum Reprod Open. 2020 Jul 31;2020(3):hoaa032. doi: 10.1093/hropen/hoaa032. eCollection 2020.
17. Legal regulations of ART
This is the list of the most important legal regulations of ART in Hungary.
1997 évi CLIV törvény az egészségügyről (Health care law)
especially 165§-185/B§
30/1998. (VI. 24.) NM rendelet
Regulations of ART procedures and legal aspects of gamete and embryo cryopreservation.
49/1997. (XII. 17.) NM rendelet
Regulation of insurance covered ART procedures.
18/1998. (XII. 27.) EüM rendelet
Regulation of tissue-, organ transplantation, storage and histopathologic exams. (1997. évi CLIV. törvény)
2013 évi V. törvény civil law affected ART.
especially a 4:98§, 4:100§, 4:108§, 4:115§
339/2008. (XII. 30.) Korm. rendelet
Government regulations about accumulating data associated with human assisted reproductive technology procedures and about the details of how to make them publicly available.
- évi CXII. törvény
- évi XLVII. törvény
About healthcare related data handling and protection.
- évi C. törvény a büntető törvénykönyvről XVI. fejezet About healthcare related clinical research.
EMMI szakmai irányelve (ART protocol)
The Hungarian protocol of infertility evaluation and assisted reproductive technology treatments. (2021.01.28-2024.01.28-ig) Egészségügyi Közlöny 2021/4. szám